It is widely recognized that efficacy and safety of many drugs and drug candidates are strongly influenced by genetic factors. Single nucleotide polymorphisms (SNPs) and more complex structural variations like insertions/ deletions or gene copy number variations in drug-metabolizing enzymes, drug transporters or targets often affect the pharmacokinetics and pharmacodynamics of a compound.
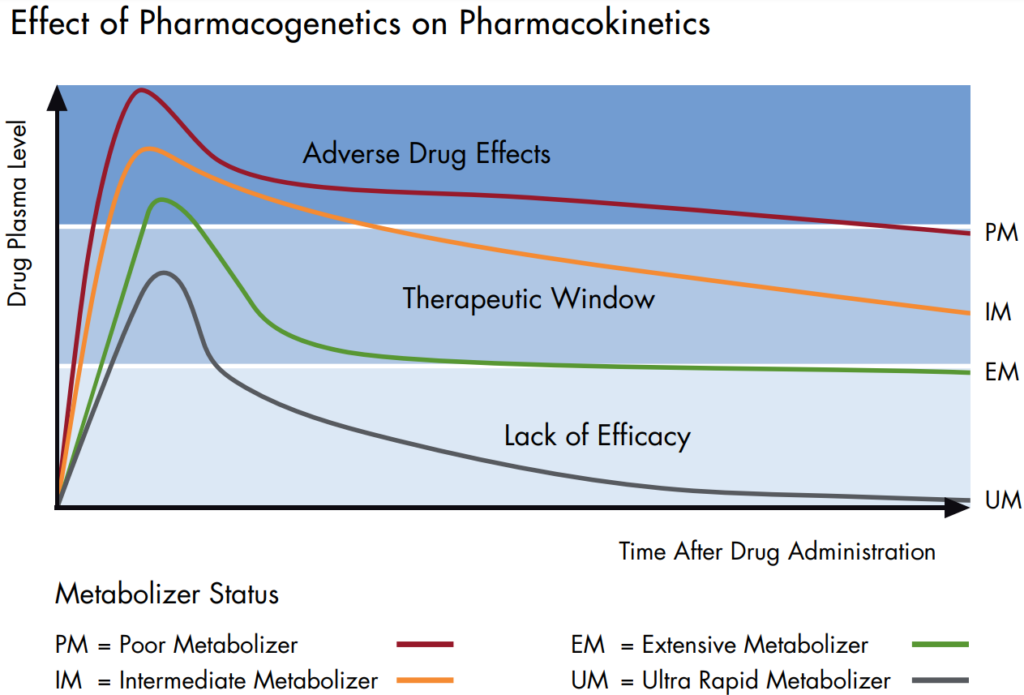
Pharmacogenetic testing in clinical studies becomes ever more significant, since this approach is able to elucidate some of the underlying causes of inter-individual variability in drug response and adverse drug reactions. Moreover, the effects of drugs or drug candidates on gene expression can be analyzed to obtain valuable insights into their mode of action (pharmacogenomic approach).
This way, the application of pharmacogenetics and pharmacogenomics is not only able to improve healthcare by sparing patients from ineffective treatments and from avoidable side effects, but also to save considerable costs for the pharmaceutical industry by streamlining clinical trials and making them more successful. Furthermore, authorities increasingly require or recommend pharmacogenetic testing as an element of the drug approval process.
Applications
- Patient stratification and specific recruitment in clinical phases
- Correlation of (adverse) drug response with genotypes
- Individualized dose adjustments
- Re-evaluation of failed drugs by inclusion of pharmacogenetic data
- Analysis of drug effects on gene expression
- Companion diagnostics
With our extensive experience and capabilities to perform variant analyses, IMGM contributes solutions on the road to a more personalized medicine. We offer a broad range of state-of-the-art technologies, analysis platforms and applications to serve your needs in pharmacogenetics, pharmacogenomics and companion diagnostics:
- Drug Metabolizing Enzymes and Transporter Analysis
- SNP Analysis
- Copy Number Variation Analysis
- Gene Expression Analysis
The human leukocyte antigen (HLA) is the human equivalent of the major histocompatibility complex (MHC). With the key role of the HLA in fine tuning the immune system, HLA genotyping is an important technique for the understanding of infectious and autoimmune diseases as well as cancer. HLA genotyping is frequently used in pharmacogenetics, clinical studies and involved in medical decisions.
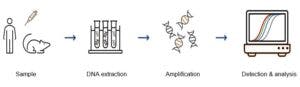
IMGM will choose from well-established typing technologies to carry out your HLA project:
- Next Generation Sequencing (NGS) Technology
-
- – Amplicon Sequencing
- – Long Range Sequencing
-
- SBT technology (sequence-based typing) – HLA typing by generic and group-specific high throughput sequencing
- PCR-SSO technology – PCR in combination with sequence-specific oligonucleotide probes coupled to beads (Luminex technology)
- PCR-SSP technology – PCR with sequence-specific primers