Project Design
Consulting and experimental design in close communication between you and your project manager at IMGM include topics like:
- Nucleic acid therapeutic characterization
- Type of analyzed DNA or RNA molecule (miRNA, mRNA, ssDNA, circular DNA etc.)
- Optimal target region for qPCR primer positioning
- Study volume and sample preparation
- Number of study animals, samples and sources (organs, body fluids, the injection site etc.)
- Optimal preparation of each sample type
- Appropriate reference materials
- Study overview and timeline
- From first sample to final results
- Realistic time frames for documentation, quality management and communication
- Validation and main study requirements
Validation Study
The method of detection is established and validated for your specific target and sample sources. Calibration curves ensure accurate absolute quantification of your target nucleic acid by qPCR.
Important aspects to consider are:
- Establishment of nucleic acid extraction
- Optimization of nucleic acid purification for each tissue type
- Risk of contamination minimized by stringent working conditions
- Quantity and quality control of every nucleic acid extract
- PCR assay design and validation
- Design of a specific TaqMan or SYBR Green assay for the nucleic acid therapeutic
- Wet-lab validation of optimal PCR conditions
- Generation of calibration curve with defined target concentrations in appropriate background matrices
- Quantification of validation study samples in triplicates
- Definition of quality control acceptance criteria
- Clear-cut threshold values for qPCR accuracy and precision, sensitivity and selectivity
- Definition of limit of detection and quantification (LOD, LOQ)
- Precise determination of nucleic acid therapeutic recovery rate and stability
- Consideration of all regulatory specifications
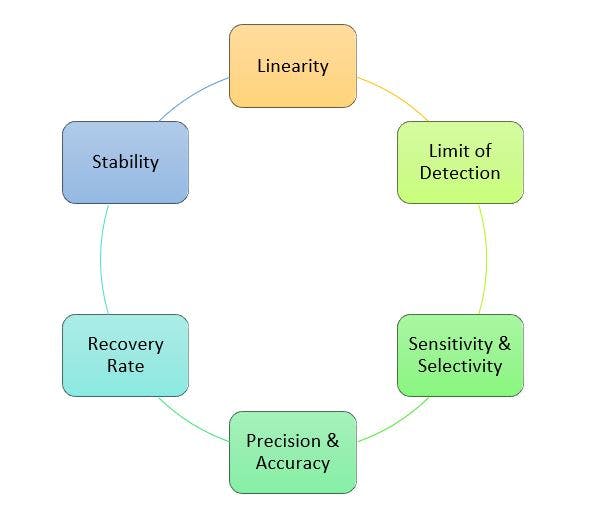
Main Study
The nucleic acid therapeutic is quantified in the main study samples applying the validated qPCR or ddPCR assay and QC criteria from the validation study. Extensive GLP-compliant documentation in submission-ready format facilitates convenient communication with regulatory authorities.