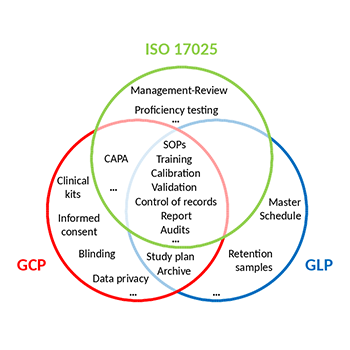
IMGM is a testing laboratory specialized in genetic analyses. We work according to international standards including Good Laboratory Practice (GLP) and the General requirements for the competence of testing and calibration laboratories (ISO°17025). To comply with Good Clinical (Laboratory) Practice (GC(L)P) IMGM follows ICH guidelines, EMA guidelines, FDA guidances and relevant legislation related to the analysis of clinical trial samples.
Following an on-site inspection, the Bavarian Health and Food Safety Authority certified that IMGM is able to conduct DNA/RNA quantification and sequencing for safety testing of medicinal products in compliance with the Principles of GLP. IMGM is included in the national GLP Compliance Programme and inspected on a regular basis.

Since 2004, IMGM holds an accreditation according to ISO 17025 awarded by the Deutsche Akkreditierungsstelle. The accreditation includes gene expression and targeted DNA analyses using next generation sequencing, as well as gene expression and SNP genotyping both using real-time PCR. Triannual re-certifications and interim surveillance audits ensure continuing compliance.

10x Genomics has appointed IMGM as “Certified Service Provider” for Single Cell Gene Expression and Immune Profiling Solution. From sample preparation to library generation to data processing, we are trained on 10x Genomics’ best practices, undergo a training and evaluation process, as well as a yearly recertification process, giving you the confidence, you need in your research results. Access the expertise of a team of highly trained scientists and bioinformaticians helping you overcome roadblocks and accelerate your research.

IMGM regularly hosts audits by customers, regulatory authorities and the accreditation body. We also kindly invite you to audit our facility and processes.
IMGM participates in external quality control programs (proficiency testing).
Note that we change schemes on purpose as to analyze different targets in consecutive proficiency testing rounds so as to cover many targets cumulatively over time.
For areas accredited proficiency testing is not offered for, we are eager to work with other laboratories and set up inter-laboratory comparisons. Please contact us!